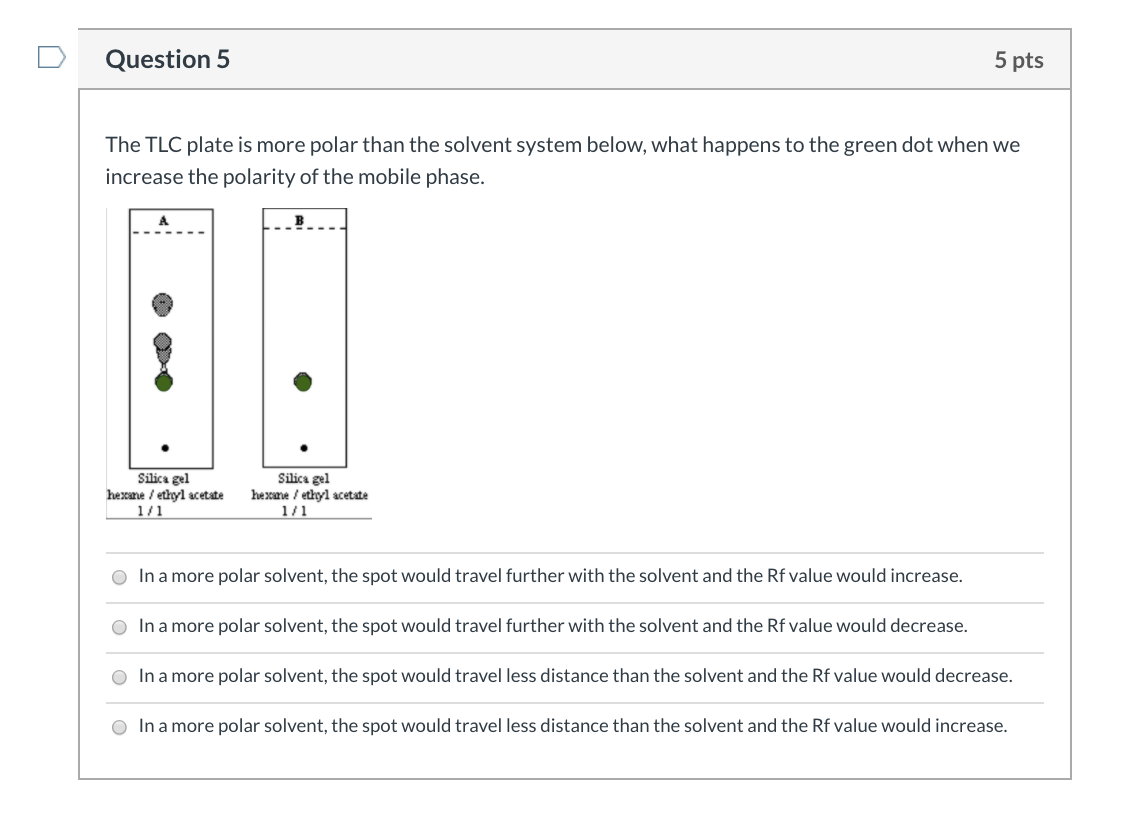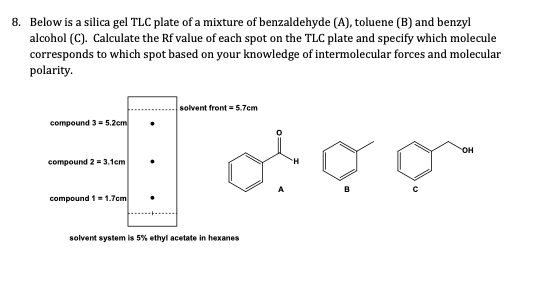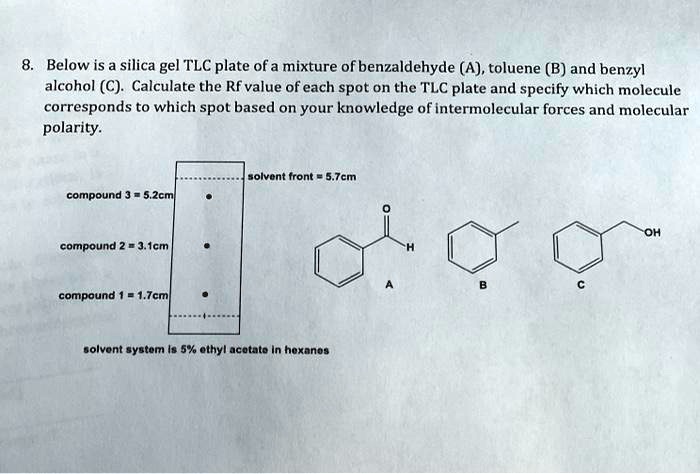
SOLVED: Below is a silica gel TLC plate of a mixture of benzaldehyde (A), toluene (B), and benzyl alcohol (C). Calculate the Rf value of each spot on the TLC plate and
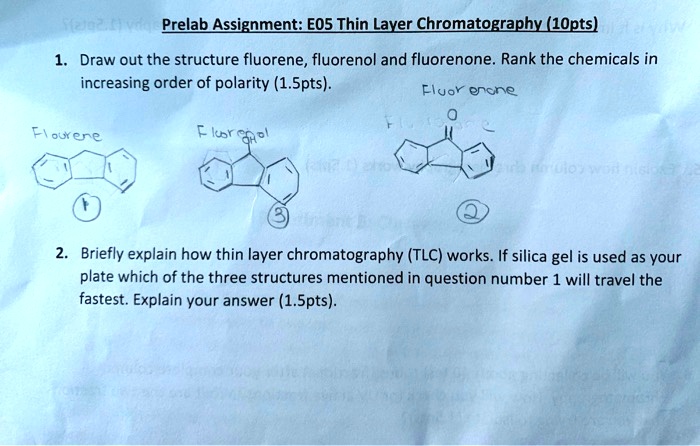
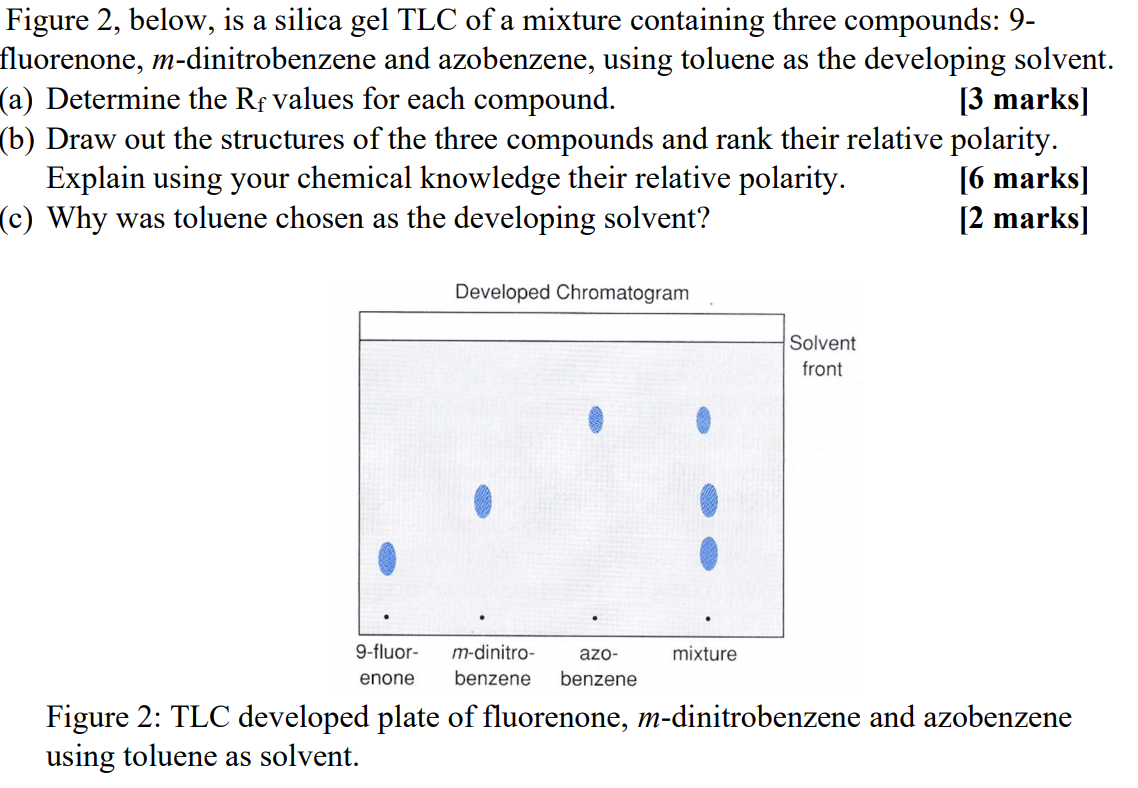
SOLVED: Prelab Assignment: EOS Thin Layer Chromatography (10 pts) Draw out the structure of fluorene, fluorenol, and fluorenone. Rank the chemicals in increasing order of polarity (10 pts). Briefly explain how thin

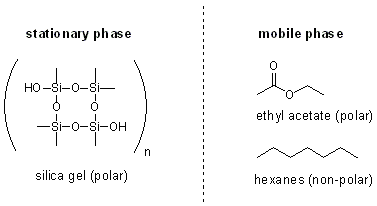
organic chemistry - How does the polarity of the eluent and sample affect the Rf value in thin layer chromatography? - Chemistry Stack Exchange