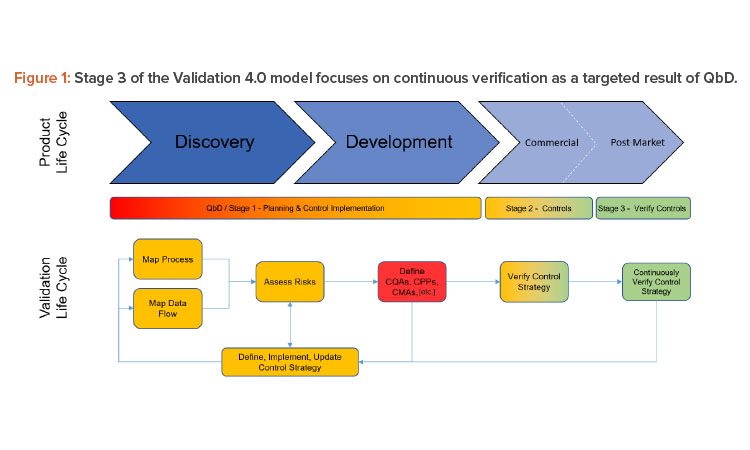
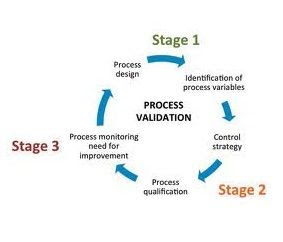
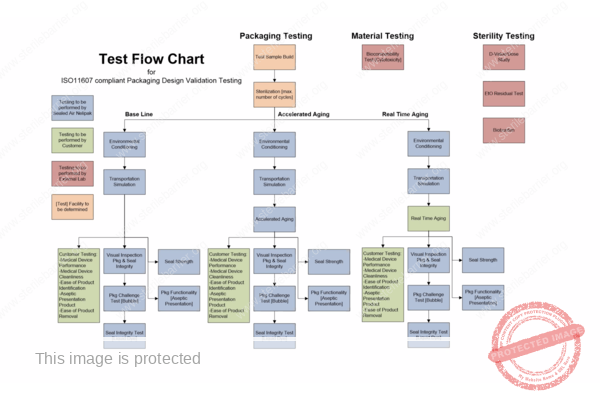
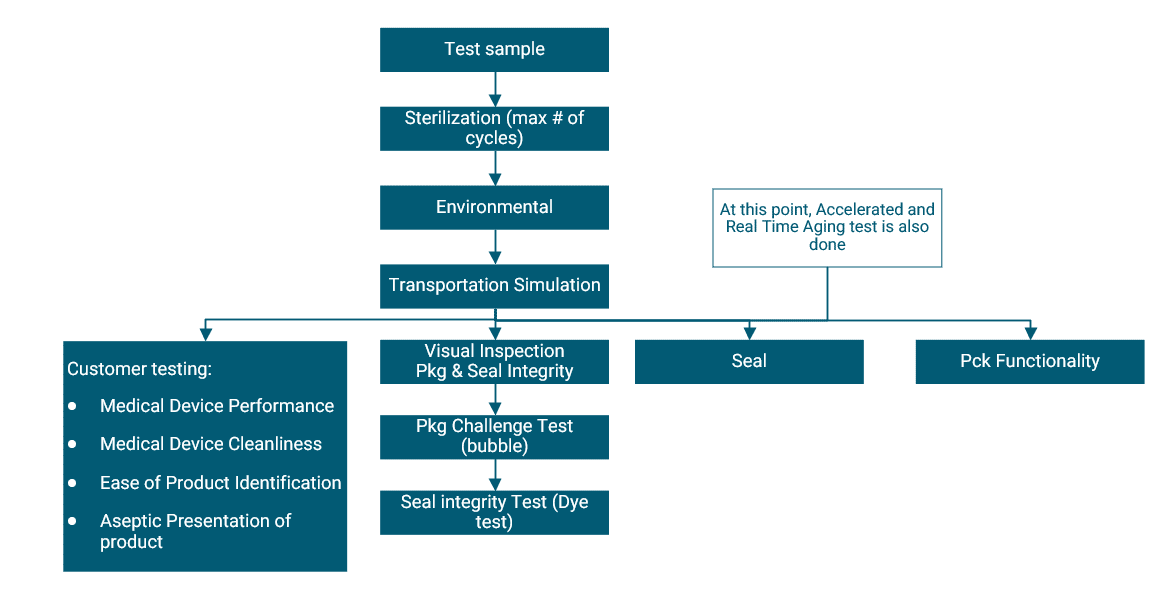
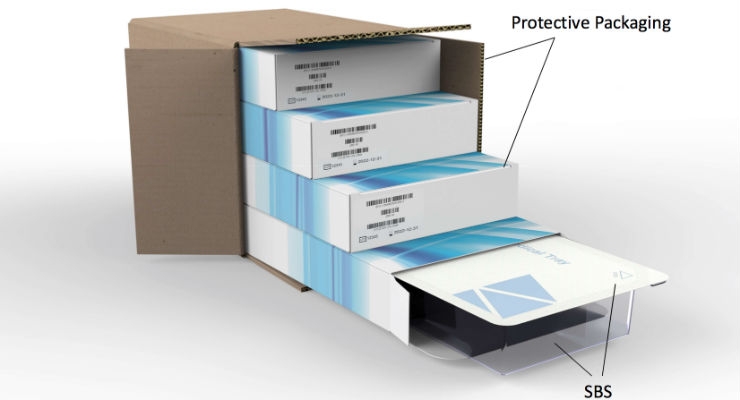
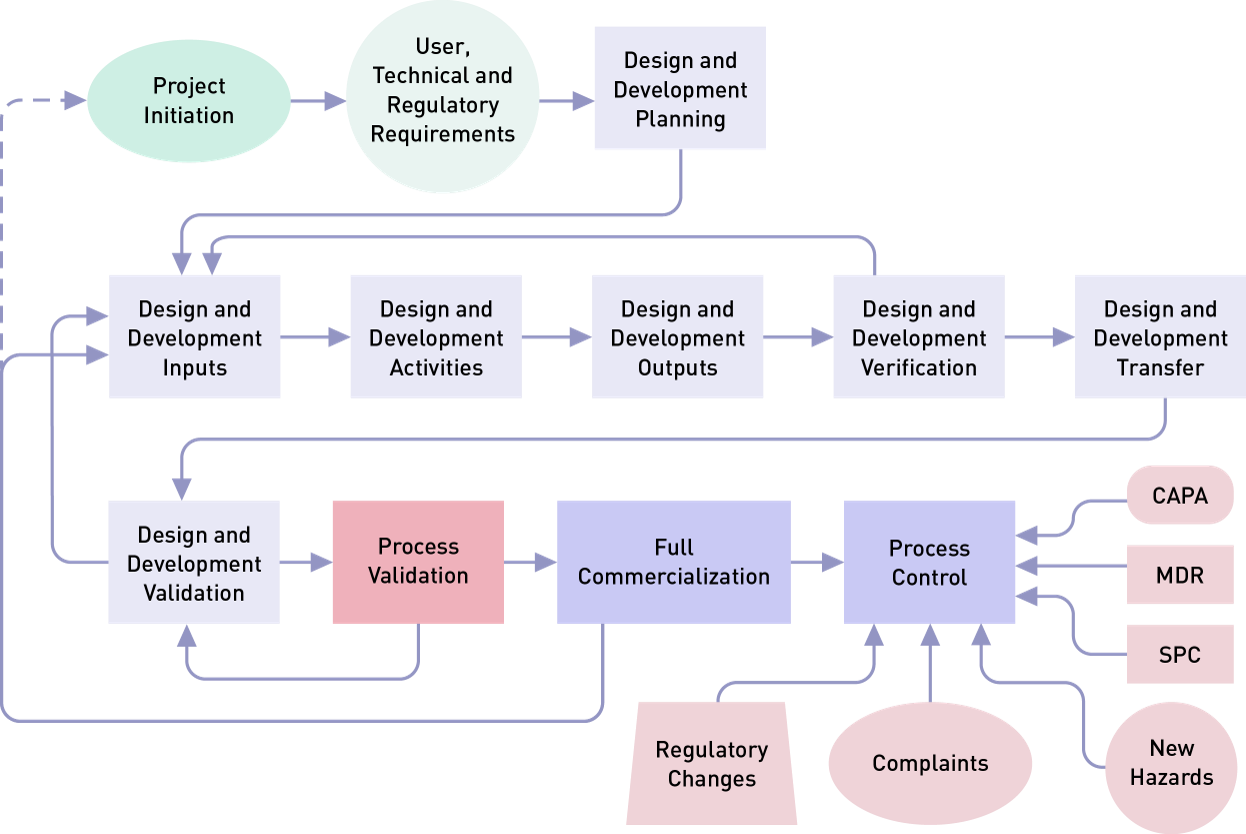
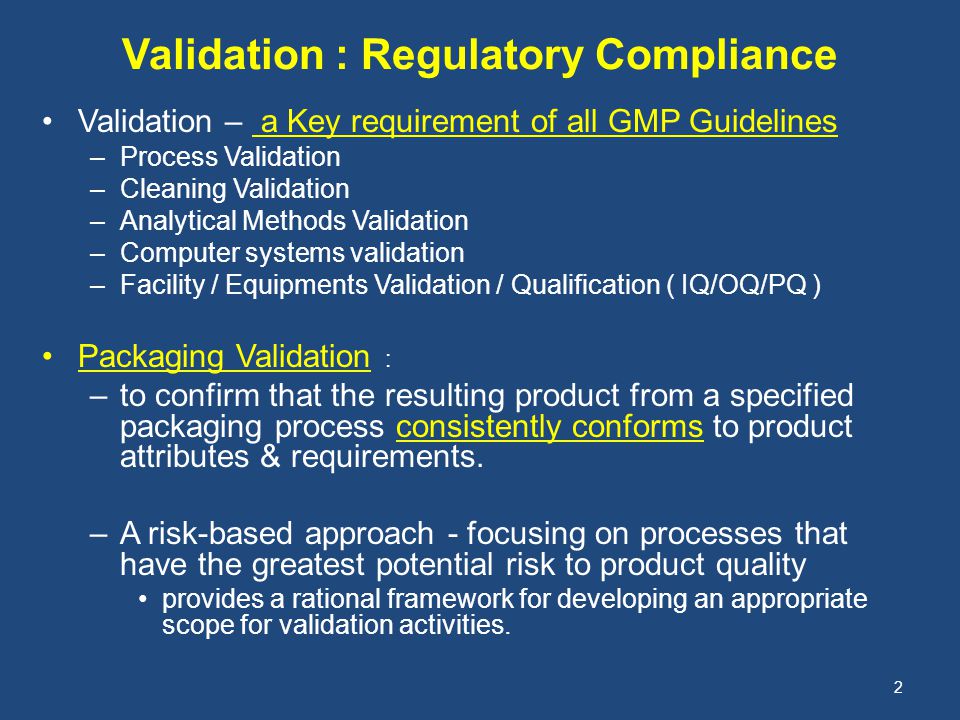
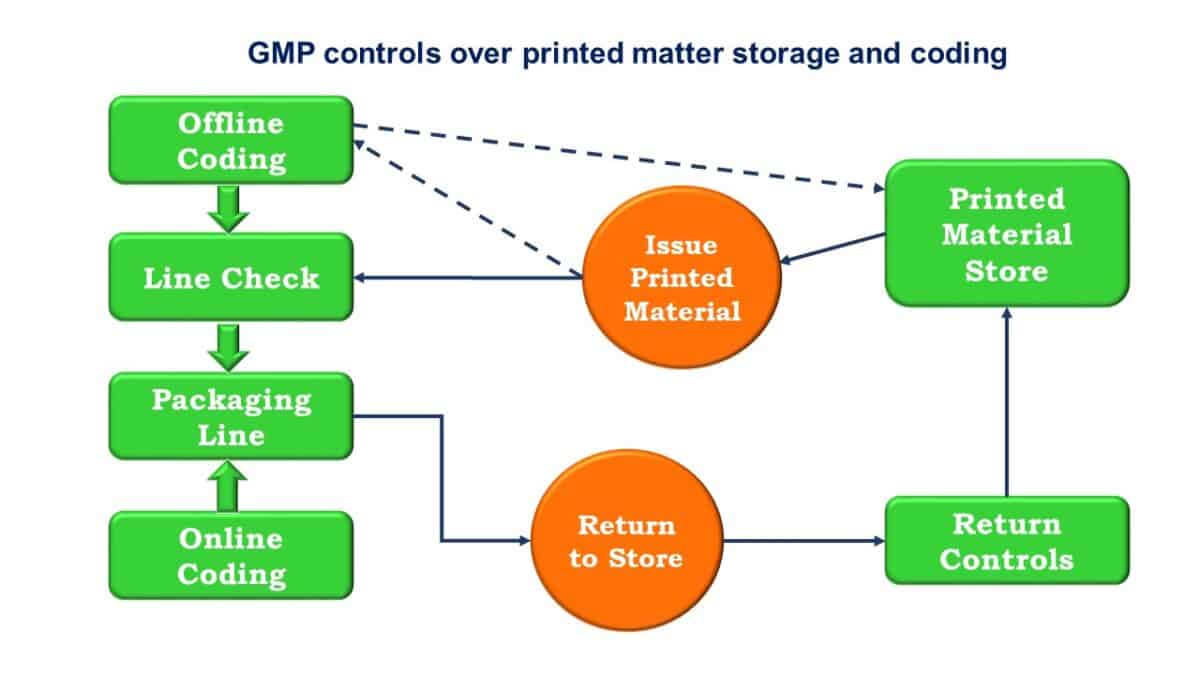
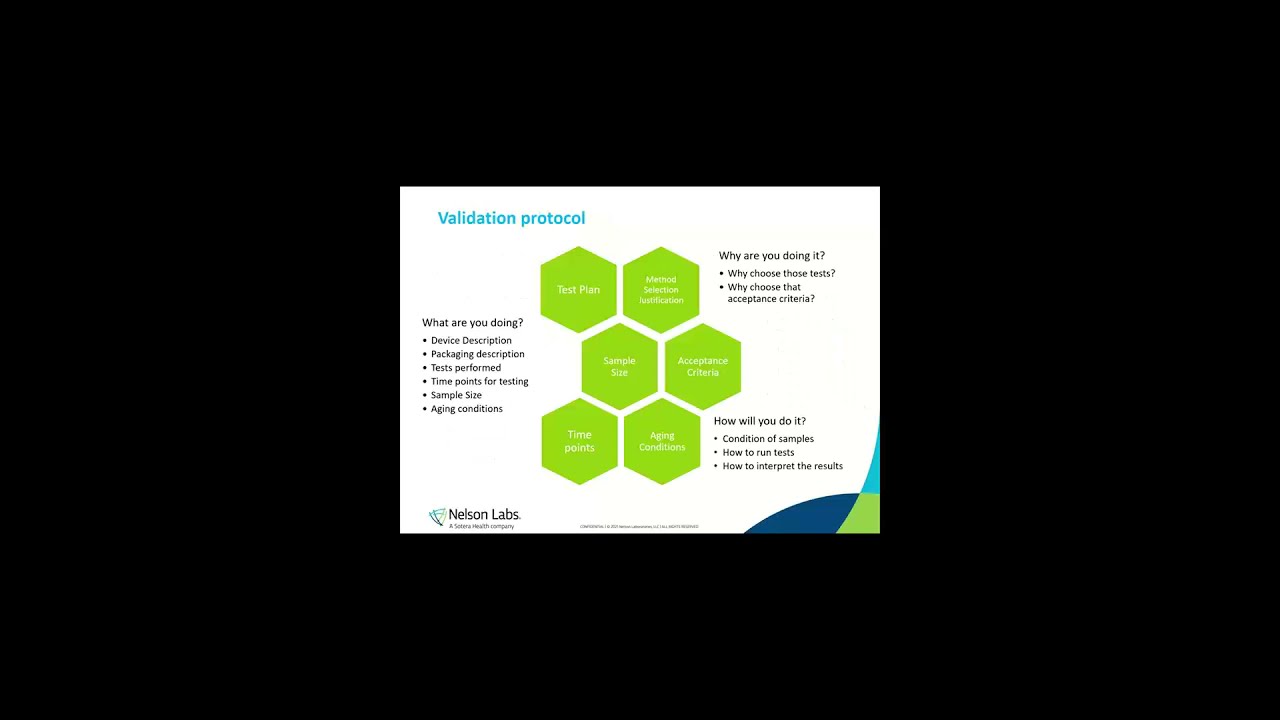
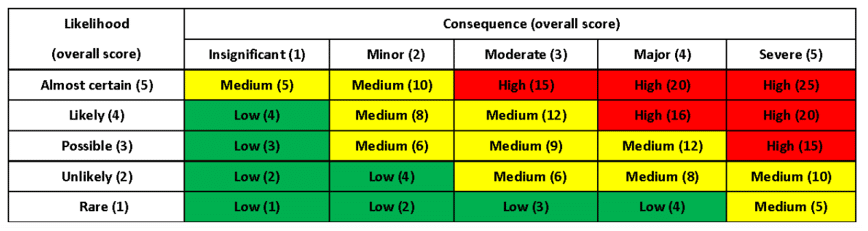
Overview of Packaging Validation for Drug Products | ISPE | International Society for Pharmaceutical Engineering

ANSI/AAMI/ISO 11607-2:2019; Packaging for terminally sterilized medical devices—Part 2: Validation requirements for forming, sealing and assembly processes